Throughout history, coffee has taken on several physical transformations, initially serving as an energy source when nomadic tribes combined coffee berries with animal fat as an early form of an energy bar. Later it was consumed as a tea, then wine, and finally to the beverage we've come to identify today. But how much of coffee’s chemical composition do we actually know?
Over the past half century scientists have made significant progress which has allowed them to unlock the nearly 1,000 compounds found in roasted coffee. In this issue of Coffee Science we’ll briefly discuss a family of compounds called ‘alkaloids’ which serve an important role in coffee’s unique chemical composition.
CAFFEINE
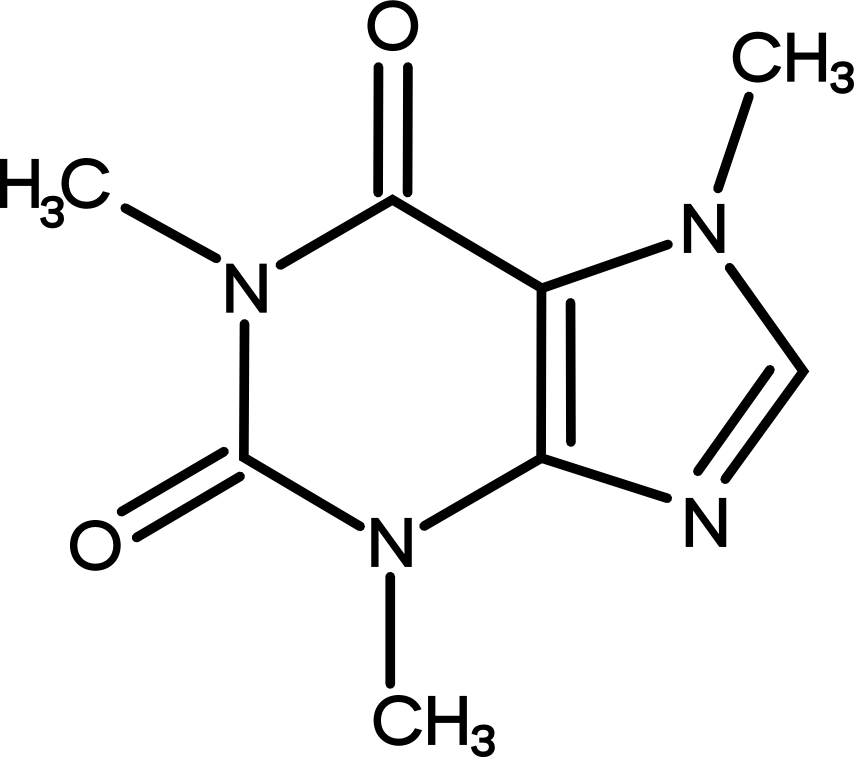
For many, coffee drinking is simply a delivery medium for a potent alkaloid we have come to identify as caffeine or otherwise known as 1,3,7 – trimethylxanthine. Although caffeine is typically associated with coffee, its production within the plant kingdom spans across numerous other plant species. Mate, for example, which is traditionally consumed in parts of Uruguay and Argentina, contains less than one percent by weight. Whereas, teas of Camellia sinesis which originated in China contain almost three times the concentration of caffeine than Arabica.
But for humans caffeine is very unique. Thus far we are the only living forms on Earth that readily seek caffeine for both its stimulatory and psychological effects. For all other life forms, caffeine is a potent toxin capable of sterilization, phytotoxicity and antifungal properties. As such scientists believe that caffeine, with its intensely bitter taste, has evolved as a primitive defense mechanism in coffee ensuring its survival in the wild for thousands of years.
It’s no surprise then, that the caffeine content of the more “robust” Robusta species is almost double that of the more delicate Arabica. The belief is that as insects attack the coffee cherry, they are deterred by the bitter taste of caffeine and simply move on to other crops.
TRIGONELLINE
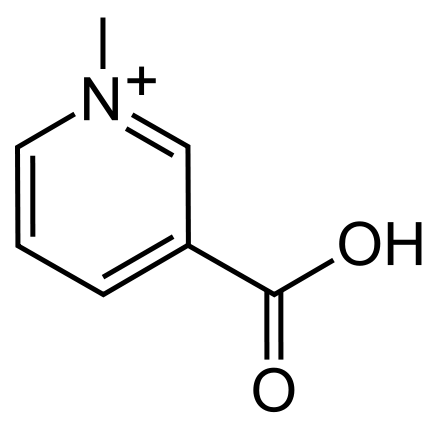
Another less known alkaloid that shadows in the light of caffeine is that of trigonelline. In Arabica coffee, trigonelline concentrations make up roughly 1% by weight with a slightly less concentration (0.7%) found its Robusta counterpart. Although its concentration is slightly less than that of caffeine, it plays a significant role in the development of important flavor compounds during roasting. But unlike that of caffeine, which survives the roasting process, trigonelline readily decomposes as temperatures approach 160°C (320°F).
Model studies have shown that at 160°C, sixty percent of the initial trigonelline is decomposed, leading for the formation of carbon dioxide, water and the development of a large class of aromatic compounds called pyridines. These heterocyclic compounds play an important role in flavor and are responsible for producing the sweet/caramel/earthy- like aromas commonly found in coffee.
Another important byproduct produced during the decomposition of trigonelline is nicotinic acid, or vitamin B3 - more commonly known as niacin. Depending on roasting conditions niacin can increase up to ten times its initial concentration, providing anywhere between 1 mg of niacin per cup for Americano type coffees and roughly two to three times this concentration in espresso type beverages. When one considers that most Americans consume about 3.2 cups of coffee per day according to the NCA (2008) – makes coffee an ample source of dietary niacin.
So far that’s great news for people with an unbalanced diet but there is another therapeutic benefit to coffee that is even more surprising. Recently Italian scientists discovered that drinking coffee may lower our incidence of dental caries. According to researchers trigonelline works by preventing the adhesion of mucus-like acid byproducts onto teeth which would otherwise lead to dental caries.
In the end, it looks like drinking a cup of coffee may not only keeps the doctor away, but the dentist as well.
Download this article in PDF format here.
Interested in more coffee science?
Make sure to sign up for our Coffee Chemistry Newsletter.
Joseph A. Rivera holds a degree in food chemistry and was formerly the Director of Science & Technology at the Specialty Coffee Association of America (SCAA). He’s the creator of the coffee science portal coffeechemistry.com and newly developed Coffee Science Certificate (CSC) program.