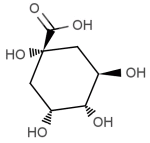
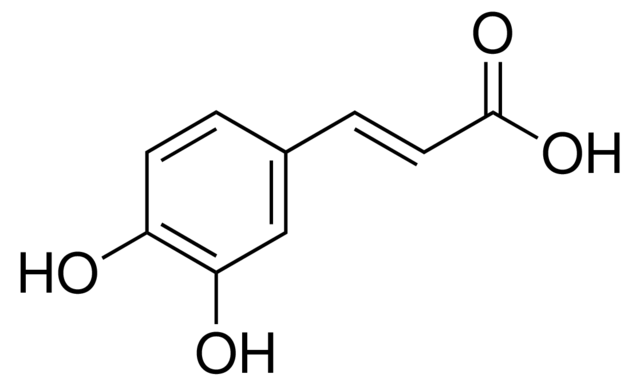
QUINIC AND CAFFEIC ACID
|
|
|
| quinic acid | caffeic acid |
As discussed last month, during the roasting chlorogenic acid progressively decomposes to yield equal units of caffeic and quinic acid. Since the reaction is temperature dependent, we can generally estimate that the formation of these secondary compounds occur at or around first crack when the bean begins to undergo significant physical changes.
Chemically, both caffeic and quinic acids are considered phenolic acids and are typically associated with astringency as found in a wide array of beverages.

Tea for example, tends to have higher levels of astringency than most other products. In coffee these effects can be seen as one goes from a light to a darker roast with corresponding levels of increasing bitterness. The sheer presence of these phenolic compounds not only effect tactile sensations, such as astringency, but over time change the level of acidity we taste in the cup as well. The phenomenon is most commonly seen during the cupping process when coffee cups are allowed to cool down in temperature.
As the coffee solution cools, a number of chemical reactions occur that ultimately raise levels of perceived acidity in the cup. The same effect is also seen to a greater extent when coffee solutions are held at elevated temperatures thereby making this reaction exponentially faster. If you've ever been served sour coffee at a local diner - it’s likely because the coffee was sitting all day on a hot plate for hours.
However, these pesky phenolics are not all bad news. According to researchers, one of the byproducts of CGA, namely caffeic acid, has been documented to have potent antioxidant and anti-inflammatory affects in vitro. That’s great news if you’re an avid drinker since coffee contains high concentration of caffeic acid. But if coffee is not your thing, you can rest assured that an occasional glass of red wine will make up for any differences as it too has been found to contain high levels of caffeic acid.
CITRIC ACID
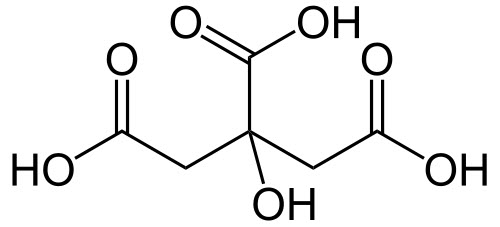
Citric Acid
2-Hydroxypropane-1,2,3-tricarboxylic acid
Another common acid that is found in coffee is one that most of us are familiar with – citric acid. Occurring naturally as part of the plant’s metabolism, citric acid plays an important role and is responsible for much of coffee’s brightness in both washed and natural coffees.
Unlike many of the other acids, citric acid is not produced during the roasting process, but rather is slowly decomposed. As coffee approaches about nine percent weight loss, there is a slow decomposition of this citric acid. Of course the exact loss depends on a number of factors, but in general a medium roasted coffee will contain about half the concentration than in its green form. Although this may not seem like much, its affect on beverage acidity is quite significant when one considers the vast array of different flavor profiles for a given coffee.
Generally medium roasted coffees will contain appreciable amounts of citric acid and will exhibit bright positive notes, but roast your coffee too light, and citric acid’s potent sourness will overpower the beverage. To demonstrate this, one needs to go no further than the kitchen counter.
The next time you’re in the kitchen, try biting into an unripe lemon and citric acid’s intense sourness will almost make your hair stand. But wait a few days and that very same lemon will become sweeter as the levels of acids and sugar change during the ripening process.

This delicate balance between these and numerous other components determine to a great extent the quality of coffee. It’s the farmer more than anyone else that plays a critical role, as simple changes in the acid/sugar ratio will ultimately have an impact on the consuming end. Hopefully, with proper education on both sides of the supply chain, we can ensure a sustainable supply of specialty coffee for years to come.
Download this article in PDF format here.
Interested in more coffee science? Make sure to signup for our Coffee Chemistry Newsletter.
Joseph A. Rivera holds a degree in food chemistry and was formerly the Director of Science & Technology at the Specialty Coffee Association of America (SCAA). He’s the creator of the coffee science portal coffeechemistry.com and newly developed Coffee Science Certificate (CSC) program.